Revolutionizing Brain Health with
AI-Powered EEG Solutions
BrainScope has developed a first-in-class, AI-driven platform that transforms brain electrical activity into a powerful assessment tool. By combining computational neuroscience with a state-of-the-art AI platform, we are unlocking the potential of EEG in ways never before imagined.
Revolutionizing Brain Health with AI-Powered EEG Solutions
BrainScope has developed a first-in-class, AI-driven platform that transforms brain electrical activity into a powerful assessment tool. By combining computational neuroscience with a state-of-the-art AI platform, we are unlocking the potential of EEG in ways never before imagined.

First AI/ML Medical Device Cleared by FDA
BrainScope’s device for head injury assessment holds the honor of being the first AI/ML-Enabled Medical Device cleared by the FDA in Neurology.
Having a proprietary device is useful in clinical settings where EEG isn't usually found, but when developing new biomarkers (pre-regulatory clearance), we can utilize EEG acquired from any third-party source through cloud or API applications.
The BrainScope Solution
BrainScope's device for head injury assessment was the first device in neurology cleared by FDA under the AI/Machine Learning-Enabled Medical Device category.
BrainScope has commercialized its first set of FDA cleared biomarkers for mild brain injury assessment with remarkable outcomes in major U.S. health systems, which include ~50% reduction in unnecessary head CTs and substantial reductions in patient length of stay in emergency departments.
BrainScope's FDA cleared biomarker for traumatically-induced brain bleed assessment could have applicability for assessment of other potentially life-threatening brain issues,
such as stroke or ARIA (abnormalities caused by amyloid-reducing Alzheimer's drugs).
BrainScope’s Large Neural Model Platform goes beyond the limits of traditional EEG and is being used to create new biomarkers
for other CNS indications across the spectrum of brain health and disease.
De-noised & Digitized EEG
Our patent-protected methodologies remove artifacts and improve the quality and interpretability of the EEG signals
Computational Model
Our computational model transforms raw EEG data into actionable insights and can incorporate other clinical datapoints in a multi-modal approach
Novel Biomarkers
Our biomarkers are easy to use, rapid, accessible at the point of care and less expensive than standard diagnostic methods such as neuroimaging.
Validated and Patented
BrainScope has developed an impressive portfolio of 83 issued patents and has commercialized three biomarkers for head injury assessment (10 regulatory clearances).
![]() NON-INVASIVE, MORE ACCESSIBLE INSIGHTS
NON-INVASIVE, MORE ACCESSIBLE INSIGHTS
The EEG Advantage
EEG has been used for over a century as a non-invasive, validated technology to provide insights into brain function. However, traditional methods rely on visual inspection, which can often miss the differences between normal and abnormal brain activity.
BrainScope has upended that model by applying machine and deep learning to EEG data, greatly expanding the clinical utility of EEG to aid in assessing and subtyping neurological diseases in ways that have never been done before. We can identify disease-relevant features within EEG starting from the earliest signs of dysfunction, well before structural brain damage occurs.

Explore
.png?width=896&height=500&name=Background%20(5).png)
BrainScope's AI solution tokenizes multi-modal neural data to create bespoke biomarkers to address a range of brain health challenges

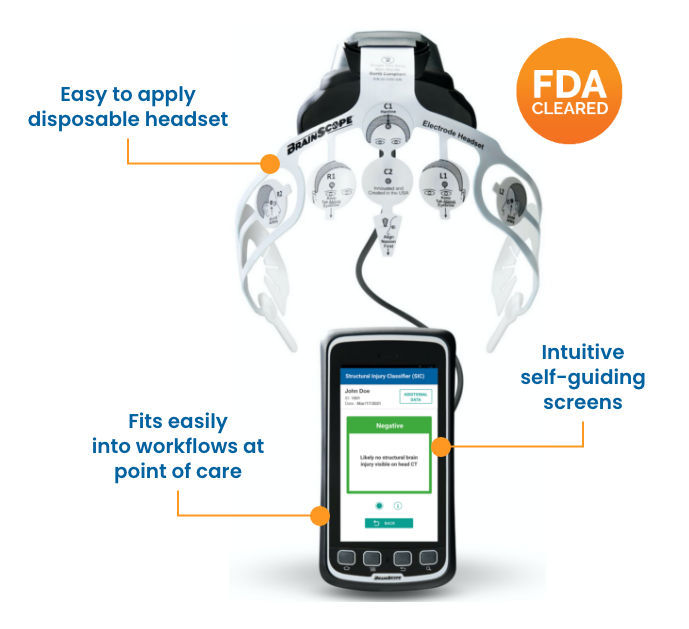
BrainScope's head injury solution is FDA-cleared, clinically validated, and trusted in hospitals and clinics for assessing brain bleeds and concussions.
.png)
Cutting-Edge Biomarker Applications
We are dedicated to creating transformative biomarkers for a range of CNS indications, including traumatic brain injury (3 FDA-cleared biomarkers), stroke, neurodegenerative diseases, behavioral health, and neuroinflammatory disorders, with applicability across:
Early Detection
Identifying brain dysfunction at its earliest stages for more timely intervention. We can also use EEG captured by third-party devices.
Tell The Reader More
Providing an objective, data-driven approach to evaluate disease progression.
Monitoring Treatment Effects
Helping determine efficacy of therapeutics and identifying potentially dangerous side effects such as ARIA associated with anti-amyloid Alzheimer's drugs.
Digital Neural Twinning and Outcome Prediction
Enabling informed decision-making and patient selection.
Subtyping Disease
Recognizing that there may be more than one form of a disease, and that treatment and treatment response may vary by subtype.
Subscribe to BrainScope News Today…
Results You Can Trust
-
Confidence to make clinical decisions
BrainScope helps clinicians confidently rule out the need for a head CT. A simultaneous concussion assessment provides objective information to aid in concussion diagnosis.
-
Fast, actionable results
Demonstrated to reduce patient length of stay in the emergency department. Prep, test, and result in under 20 minutes. -
Integrate with Ease
Get your entire team up and running with the help of BrainScope Academy and our experienced clinical team.