Improve the value of care with BrainScope
Clinical experience demonstrates...
40-60% reduction in low-value head CTs
- 50% reduction in patient LOS
50% improvement in flagging patients for follow up care
100% reported patient satisfaction
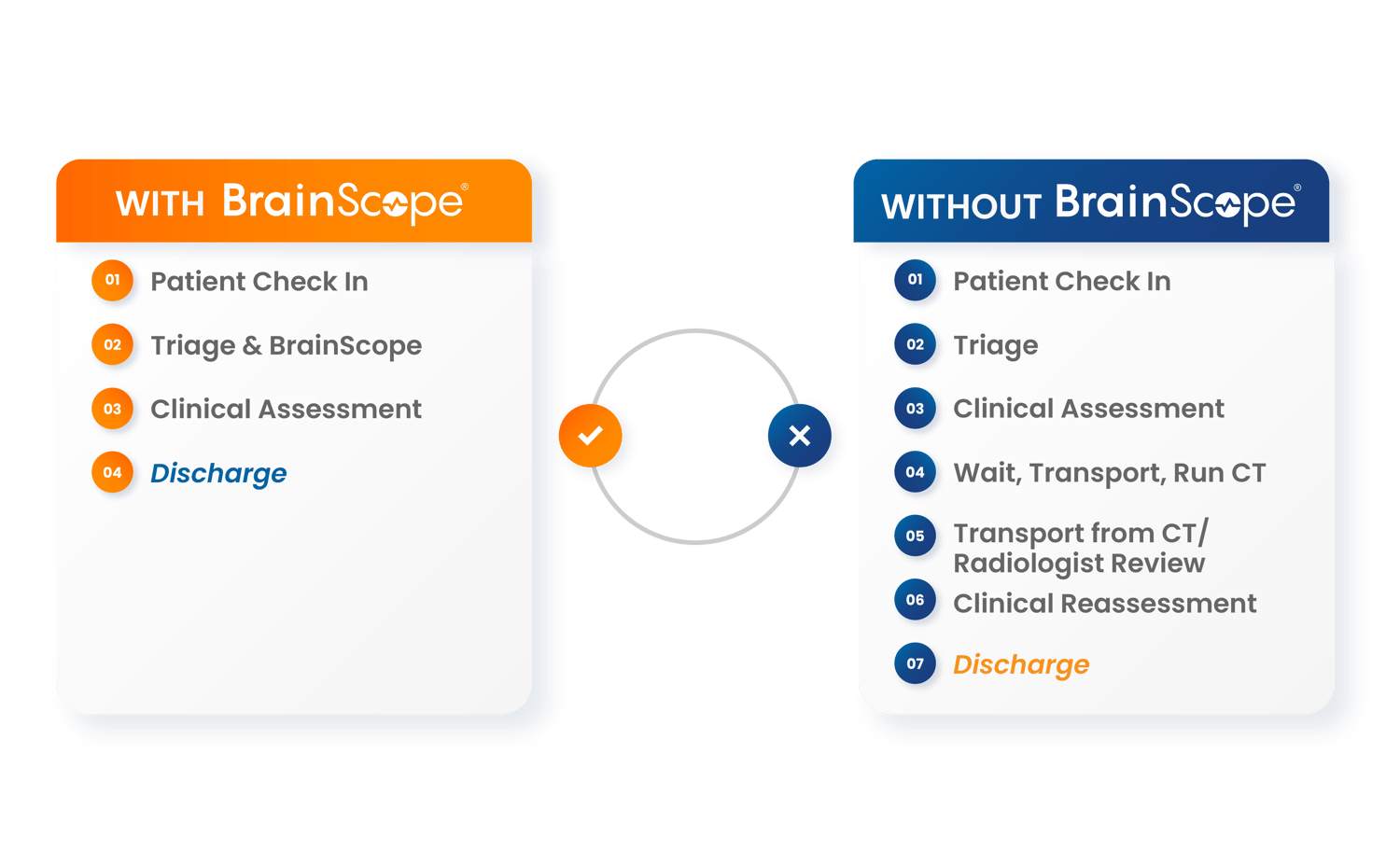
Save time, see more patients
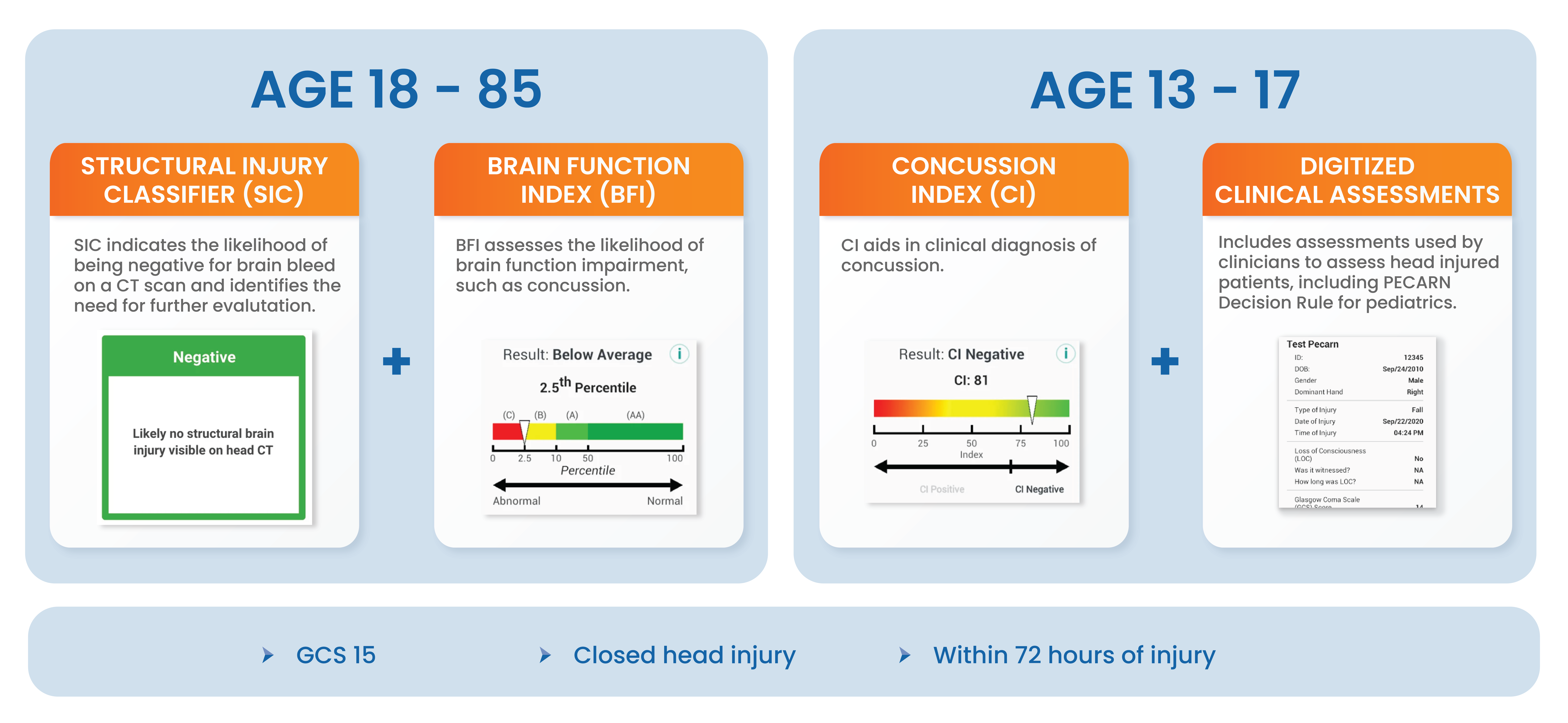
Who is BrainScope for?
BrainScope is not intended as a stand-alone diagnostic or to be used as a substitute for a head CT scan. Click here for complete indications for use.
Who is BrainScope for?

Structural Injury
Classifier (SIC)
The Structural Injury Classifier (SIC) indicates the likelihood of being negative for brain bleed on a CT scan and identifies the need for further evaluation.
- Ages 18 – 65 years, GCS 15
- Within 72 hours of injury

Brain Fuction
Index (BFI)
BrainScope’s Brain Function Index (BFI) is expressed as a percentile of a normal population and assess the probability of brain function impairment, such as concussion.
- Ages 18 – 65 years, GCS 15
- Within 72 hours of injury

Concussion
Index (CI)
The Concussion Index (CI) aids in identifying the likelihood of concussion.. The CI assessment can aid in clinical decision making at the time of injury
- Ages 13-25 years, GCS 15
- Within 72 hours of injury
Head injury triage in three easy steps

Prep
Prepare the patient's skin and apply the peel and stick electrodes with on-device guidance.

Test
Run the assessment with a self guided process and receive real-time feedback on brain electrical activity recording.

Result
Get immediate, objective results on the device and as PDF for upload to EMR.
References
Naunheim, R.,et al. (2019). Reduction in unnecessary CT scans for head-injury in the emergency department using an FDA cleared device.The American Journal of Emergency Medicine,37(10), 1987-1988.
Sheppard JP, Nguyen T, Alkhalid Y, Beckett JS, Salamon N, Yang I. Risk of Brain Tumor Induction from Pediatric Head CT Procedures: A Systematic Literature Review. Brain TumorRes Treat. 2018 Apr;6(1):1-7. doi: 10.14791/btrt.2018.6.e4. PMID: 29717567; PMCID: PMC5932294.
Michelson, E. A., et al. (2018). Emergency department time course for mild traumatic brain injury workup. Western Journal of Emergency Medicine,19(4), 635
ACEP Ambulatory Payment Classification FAQ, https://www.acep.org/administration/reimbursement/reimbursement-faqs/apc-ambulatory-payment-classifications-faq/ (accessed August 1, 2022)
Statistical Trends in the Emergency Department (ACEP) https://www.acepnow.com/article/statistical-trends-of-diagnostic-testing-in-the-emergency-department/{Accessed August 17, 2022)
DeAngelis, J., Lou, V., Li, T., Tran, H., Bremjit, P., McCann, M., ... & Jones, C. M. (2017). Head CT forminor head injury presenting to the emergency department in the era of choosing wisely.Western Journal of Emergency Medicine,18(5), 821.
Clay, MS, Clinical Utility of an EEG Based Biomarker for the Triage of Head Injured Patients in the ED: INOVA Pilot Study BrainScope White Paper, August 2021
CMS - Search the Physician Fee Schedule, https://www.cms.gov/medicare/physician-fee schedule/search (accessed August 1, 2022)
ACEP Ambulatory Payment Classification FAQ, https://www.acep.org/administration/reimbursement/reimbursement-faqs/apc-ambulatory-payment-classifications-faq/ (accessed August 1,2022)
Bentkover, JD et. al Economic Impact on the Healthcare Systemusing an FDA-Cleared Mild Brain Injury/Concussion Assessment Device BrainScope One Economic Analyzer Model (BEAM):White Paper, 2018




